Ongoing Research
Computational Combustion Modeling | Combustion-generated Pollutants | Atmospheric Soot | High-performance Computing
Multiscale modeling of soot
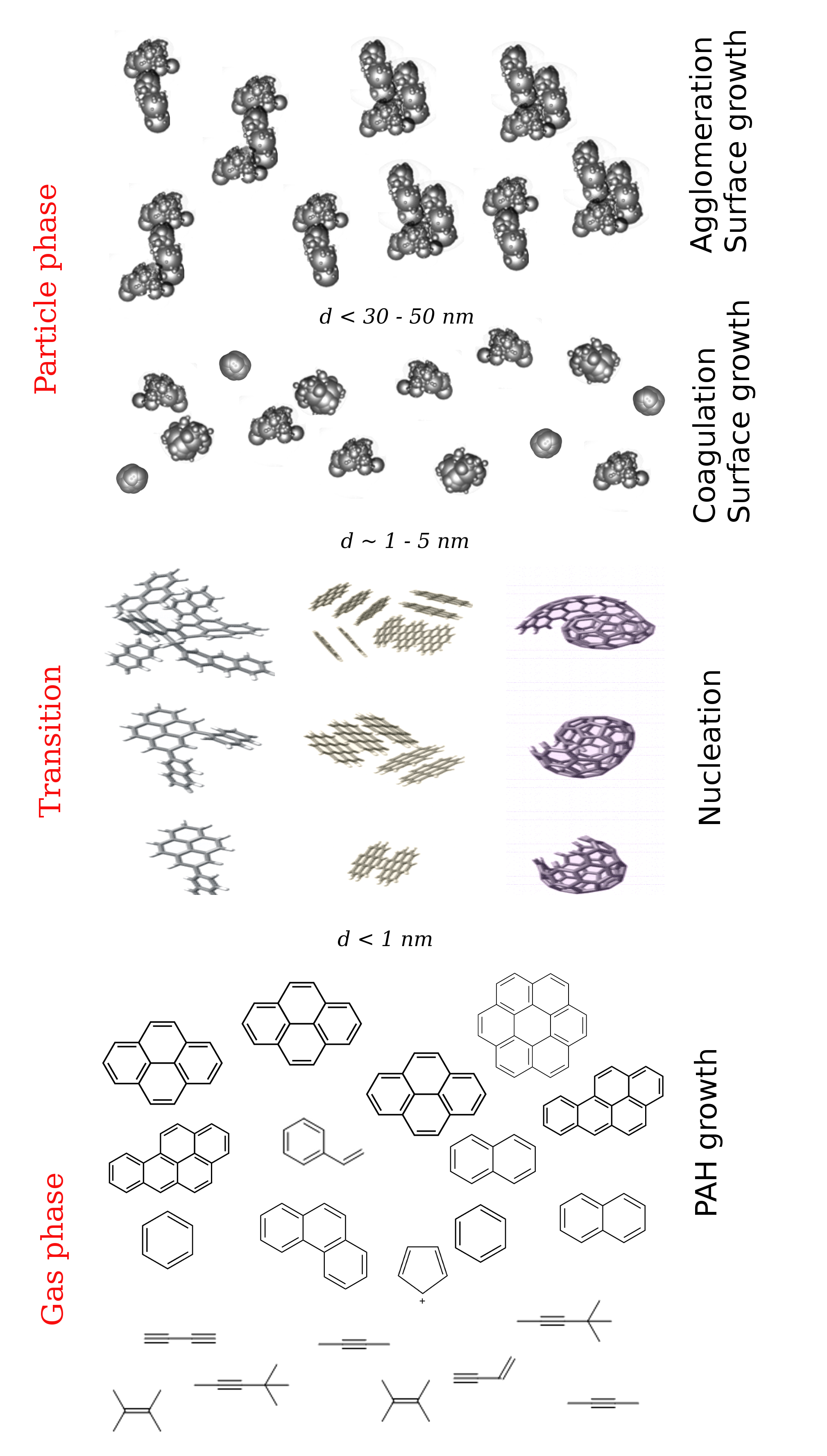
The exact process of soot formation is yet to be fully understood. This is partly because of experimental uncertainty, partly because of the complex physico-chemical processes spanning multiple scales leading to soot formation. Understanding the physics and chemistry of soot formation will require understanding the soot nucleation from an atomistic standpoint as well as exploring the evolution of soot from an aerosol-dynamics viewpoint.
The transition from gaseous combustion products to a solid particulate phase starts via formation of large aromatic molecules aka polycyclic aromatic hydrocarbons (PAHs). They interact with one another and with other small species, and at one point they — by processes that are still not understood — form what can be termed as the first soot particle. Its diameter is close to a nanometer.
The incipient soot particles then grow and evolve due to physical interactions (e.g., Brownian collision) and chemical reactions (e.g. surface reactions) as they travel through the combustion gases. The exact nature of these physico-chemical processes — another poorly understood phenomena — controls how the shape, size, and properties of the final soot particles are going to be as they escape the combustion domain.
Recent CCL Publications
- The coalescence of incipient soot clusters. Sharma, A.; Mukut, K., M.; Roy, S; and Goudeli, E. Carbon, Accepted, 2021.
- A Closer Look into the Formation of Soot Particles: A Molecular Dynamics Study. Mukut, K., M.; Sharma, A.; Goudeli, E.; and Roy, S. In 12th US National Combustion Meeting, 2021.
- Effect of O2 Concentration in Ambient Mixture and Multiphase Radiation on Pollutant Formation in ECN Spray-A. Mukut, K., M.; and Roy, S. Combustion Theory and Modelling, 24(3): 549-572. 2020.
- A Systematic Comparison of Detailed Soot Models and Gas-phase Chemical Mechanisms in Laminar Premixed Flames. Roy, S., P.; and Haworth, D., C. Combustion Science and Technology, 188(7): 1021-1053. 2016.
Researchers
- Khaled Mosharraf Mukut
- Eduardo Carrasco
Supporting organizations

|

|








